- HOME
-
ABOUT
- History
- Vision, mission, strategy
- Organization structure
- Quality management system
- Plan & Performance
- Annual Report
- Award
- Related Laws
- Integrity organization
- Integrity and Transparency Assessment (ITA)
- Business Continuity Plan (BCP)
- Website Policy
- News & Activities
-
Services
- Biopharmaceuticals and ATMPs products
- Laboratory Proficiency Testing
- Medical plastic/rubber analysis
- Food Supplement & Coffee
- Drug analysis
- Quality Assurance of Medicines Program
- Reference substance
- Pharmacopoeia of Thailand
- Narcotic analysis
- Collaborating Center for Cannabis Laboratories
- Test kit
- Service manual & rate
- Server form
- Research
- Knowledge
- Seminar
- FAQ
- Contact Us
- HOME
- ABOUT
- News & Activities
-
Services
- Biopharmaceuticals and ATMPs products
- Laboratory Proficiency Testing
- Medical plastic/rubber analysis
- Food Supplement & Coffee
- Drug analysis
- Quality Assurance of Medicines Program
- Reference substance
- Pharmacopoeia of Thailand
- Narcotic analysis
- Collaborating Center for Cannabis Laboratories
- Test kit
- Service manual & rate
- Server form
- Research
- Knowledge
- Seminar
- FAQ
- Contact Us
- HOME
- ABOUT
- Organization structure
ABOUT
Organization structure
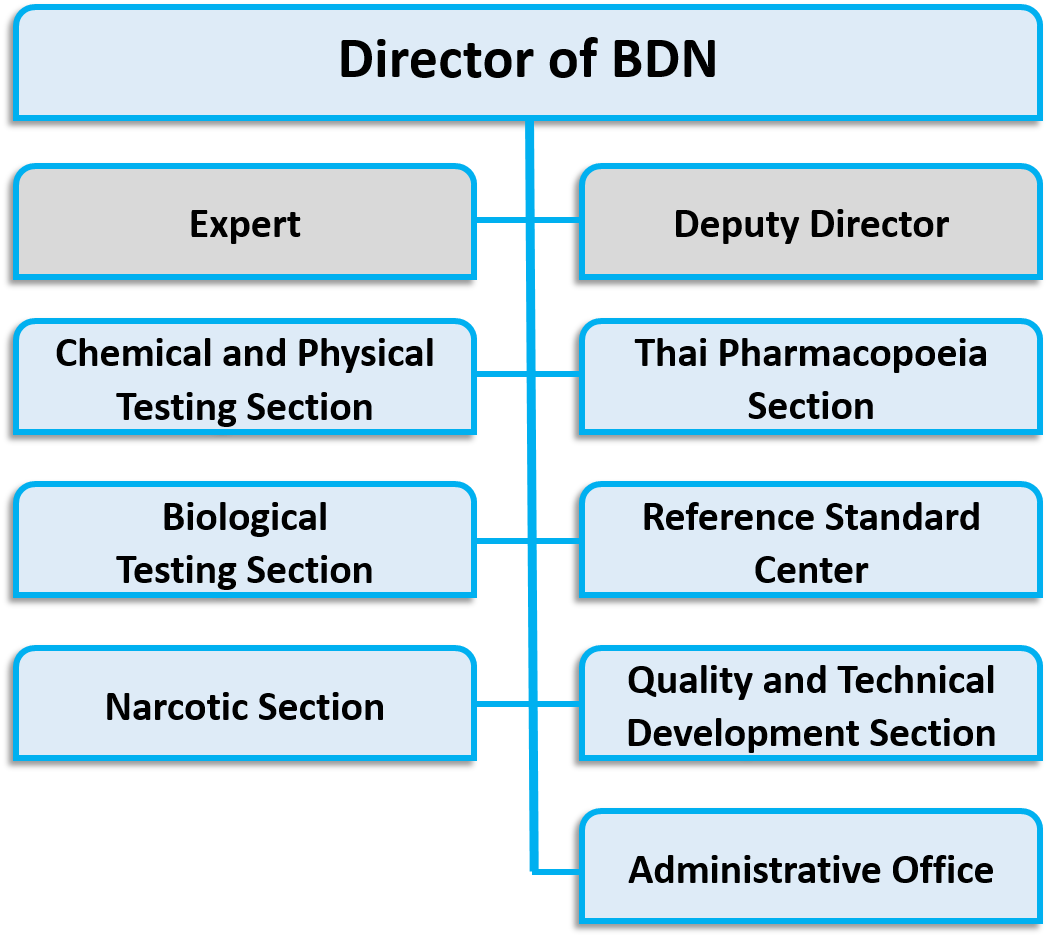
Responsibilities of sections
1. Chemical and Physical Testing Section
Chemical and Physical Testing Section is divided into four laboratories: Chemical and Physical Testing Laboratory 1, 2, 3 and 4. Their responsibilities are as follows:
- perform both physical and chemical analysis of pharmaceutical products
- testing of imported active pharmaceutical ingredients (API) to ensure their compliance with established specification
- conduct research programme to develop new testing methods
- develop and transfer analytical technology to government and private laboratories
- develop test kit for screening test of drug substances and adulterations
- qualitative analysis of seized materials
2. Narcotic Section
Narcotic Section consists of three laboratories: Narcotic Laboratory 1, 2 and 3. Their responsibilities are as follows:
2.1 Narcotic Laboratory 1
- confirmatory test of narcotics in urine specimens
- develop standard test methods
- develop test kit for screening test of psychotropic and narcotic substances
- conduct survey for drug abuse
2.2 Narcotic Laboratory 2
- qualitative and quantitative analysis of illicit drugs
- develop standard test methods
- develop test kit for screening test of psychotropic and narcotic substances
- conduct survey for drug abuse.
2.3 Narcotic Laboratory 3
- chemical analysis for psychotropic and narcotic substances used as medicines
- qualitative and quantitative analysis of illicit drugs
- develop standard test methods
- develop test kit for screening test of psychotropic and narcotic substances
- conduct survey for drug abuse.
3. Biological Testing Section
Biological Testing Section consists of three laboratories: Biological Testing Laboratory 1, 2 and 3. Their responsibilities are as follows:
3.1 Biological Testing Laboratory 1
- analysis of antibiotics using microbiological methods
- contamination testing of analysis of non-sterile pharmaceutical products
- sterility testing of parenteral products
- conduct research programme to develop new testing methods
- develop and transfer analytical technology to government and private laboratories
3.2 Biological Testing Laboratory 2
- research and development on biopharmaceutical products
- development of standard method for quality control of biopharmaceutical products including biosimilars
- post-marketing surveillance of biopharmaceutical products including biosimilars
- provide technical support and training to government and private sectors
3.3 Biological Testing Laboratory 3
- pyrogen / bacterial endotoxin testing of parenteral products
- biocompatibility testing of plastics and polymers
- conduct research programme to develop new testing methods
- develop and transfer analytical technology to government and private laboratories
4. Quality and Technical Development Section
- prepare strategic plan, action plan, budget, personnel development, coordinate and organizes local as well as international training programmes
- coordinate quality assurance activities to ensure that all laboratory activities are performed according to laboratory quality system
- planning for instrument calibration according to quality system
- IT programme developing for the Bureau
- coordinate the proficiency testing programme to assess laboratory performance
5. Thai Pharmacopoeia Section
- study on various aspects of drugs and preparations e.g. their pharmacology, chemistry, including testing the methods for identification and assays in order to establish their specifications in the Thai Pharmacopoeia
- publish the successive volume of Thai Herbal Pharmacopoeia
- issue the Pharmacopoeial Newsletter
6. Reference Standard Center
- establish drug and narcotic reference substances for locally use and within ASEAN member countries
- training center for the production of ASEAN reference substances for member countries
7. Administrative Office
- receive and dispatch official papers
- type analytical reports and official correspondence
- filing and archiving documents
- procurement of laboratory, scientific as well as office supplies
- maintain accounting, administrative budget management and personnel services
- serve as central registry for incoming sample

